December 12, 2025 (Current Version)
December 12, 2025
December 12, 2025
The world of medical weight loss has been transformed in just a few years. Medications like Wegovy, Mounjaro and Zepbound have captured headlines with their ability to help people lose double-digit percentages of body weight. But now, Eli Lilly’s newest experimental drug, retatrutide, may be starting an entirely new chapter.

In December 2025, Lilly released the first Phase 3 data from its TRIUMPH-4 trial. The results were dramatic. Retatrutide delivered more body-weight reduction than any medication ever tested, even surpassing Lilly’s own Zepbound/Mounjaro and Novo Nordisk’s Wegovy. It also significantly reduced knee arthritis pain, which is incidentally a major challenge for people living with obesity.
But as powerful as it is, the drug also brought higher-than-expected side effects and notable dropout rates, raising questions about who will ultimately benefit the most once the drug reaches the market.
What Is ‘Triple G’
“Triple G” is the nickname for retatrutide and refers to the three metabolic hormone pathways the drug activates:
- GLP-1 (Glucagon-Like Peptide-1)
Reduces appetite, slows stomach emptying, improves blood sugar regulation. - GIP (Glucose-Dependent Insulinotropic Polypeptide)
Enhances insulin response, supports appetite control, may reduce nausea at lower doses. - Glucagon
Increases energy expenditure (boosted calorie burn), helps regulate blood sugar, and may enhance fat breakdown.
Record-Setting Weight Loss in 68 Weeks
The TRIUMPH-4 Phase 3 trial enrolled 445 adults with both obesity and knee osteoarthritis. Over 68 weeks, participants were randomized to either retatrutide or placebo. Weight loss was the primary endpoint and the results were stronger than nearly anyone expected.
At the highest dose of retatrutide:
- Average body-weight loss for patients who completed the study was 28.7%
- Average body-weight loss among all participants (including dropouts) was 23.7%
To put this into perspective:
- Wegovy’s typical weight loss is around 15%
- Zepbound’s/Mounjaro’s Phase 3 results showed 22.5%
- Retatrutide’s own Phase 2 produced 24.2% at 48 weeks
- Many bariatric surgery patients lose 25–35% of body weight
This means retatrutide’s weight loss in Phase 3 is approaching the lower end of bariatric surgery, while requiring only a once-weekly injection.
Even More Striking: How Many People Achieved Deep Weight Loss
One of the most important findings wasn’t just the average weight loss — it was how many people achieved very large reductions in body weight:
- 39% of participants lost 30% or more
- 24% lost at least 35% of their starting body weight
These levels of fat loss have never been seen before with medication. For many people, losing 30–35% of body weight can mean reversing obesity-related conditions, improving mobility, and reducing long-standing knee or back pain.
Dramatic Knee Pain Relief – A Key Bonus
Although retatrutide is being developed primarily as a weight-loss drug, TRIUMPH-4 also measured its effects on knee osteoarthritis pain, a common issue for people with excess weight.
Across the study:
- Patients on the highest dose had a 75.8% reduction in knee pain
- More than 12% reported being completely pain-free
- Over two-thirds experienced at least a 70% improvement
- Systolic blood pressure dropped by 14 mmHg, an additional cardiovascular benefit
For people with obesity who struggle with daily movement or have been told they may eventually need a knee replacement, these improvements could be life-changing. Pain relief also makes it easier for patients to increase activity levels, an important part of maintaining long-term weight loss.
Why Retatrutide Works: The First “Triple G” Approach
Retatrutide is the first drug to combine three different metabolic hormone pathways:
- GLP-1 – reduces appetite and slows digestion
- GIP – enhances insulin sensitivity and may stabilize appetite signals
- Glucagon – increases energy expenditure and may help burn fat more efficiently
- Wegovy and Ozempic activate GLP-1 only.
- Zepbound (tirzepatide) activates GLP-1 + GIP.
- Retatrutide adds glucagon, creating what researchers call a “Triple G” or “tri-agonist.”
The addition of glucagon appears to be a key reason why retatrutide produces such significant weight loss, although scientists are still investigating the exact mechanism. Some believe the combination leads not only to lower appetite but also to higher calorie burn, giving it a dual benefit that current GLP-1 medications cannot match.
Side Effects: The Biggest Question Mark
As impressive as the efficacy data are, retatrutide did not come without challenges. The most concerning issue raised by the Phase 3 results was tolerability, particularly at higher doses.
Discontinuation Rates Were Higher Than Expected
Patients stopped treatment because of side effects at the following rates:
- 18.2% on the highest dose
- 12.2% on the 9 mg dose
- 4% on placebo
By comparison:
- Zepbound’s/Mounjaro’s discontinuation rate was 6.2%
- Retatrutide’s Phase 2 discontinuation reached 16%
This suggests that while retatrutide produces massive weight loss, it may also present more difficulty for some patients.
Most Common Side Effects
Retatrutide’s side effects were similar to other GLP-1-based drugs, but slightly more pronounced:
Because the drug works on multiple hormone systems, it may place more metabolic stress on the body, particularly early in treatment.
A Unique Side Effect: Dysesthesia
One unusual symptom stood out:
- Dysesthesia, an unpleasant skin sensation, occurred in up to 20.9% of high-dose users
- Placebo rate: 0.7%
Most cases were mild, and few caused patients to stop treatment, but this is something clinicians will watch closely as more data emerge. Because dysesthesia is less common among dual-agonist drugs, the addition of glucagon may be influencing nerve sensitivity.
Lower BMI = Higher Dropout
Interestingly, people with lower starting BMIs were more likely to stop the drug. Many discontinued due to what they perceived as excessive or rapid weight loss. When excluding those with lower baseline BMIs, discontinuation rates became similar to what’s seen with other obesity medications.
This may mean retatrutide is particularly well-suited for:
- People with severe obesity
- Individuals aiming for very large weight loss
- Patients whose health risks are high enough to justify a stronger medication
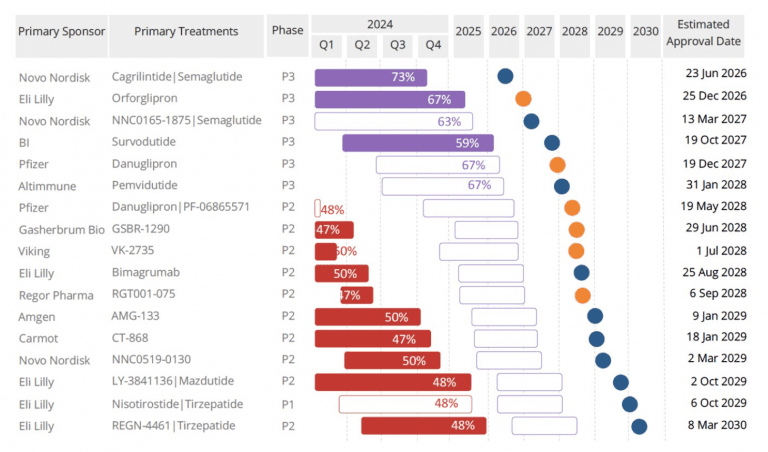
How Retatrutide Fits Into the Competitive Landscape
Lilly is aggressively expanding its obesity drug portfolio. Retatrutide follows:
- Zepbound/Mounjaro, a major global success
- Orforglipron, a once-daily oral drug completing Phase 3 in 2025
The company has invested heavily in manufacturing, including major U.S. facilities, to meet massive demand for weight-loss medications.
Competitor Novo Nordisk is advancing therapies as well, including CagriSema , a combination of semaglutide (the active ingredient in Wegovy) and amylin. But so far, their trials have achieved 22–23% weight loss, impressive but significantly below retatrutide’s upper range.
This means retatrutide could become the undisputed top-tier medication for individuals needing substantial weight reduction.
What Comes Next: More Trials in 2026
Lilly will complete seven additional Phase 3 trials in 2026. These include studies of retatrutide in:
- Obesity
- Type 2 diabetes
- Obstructive sleep apnea
- Chronic low back pain
- Cardiovascular outcomes
- Renal outcomes
- Liver disease (MASLD)
More than 5,800 people are enrolled across the Phase 3 program, making retatrutide one of the most thoroughly tested weight-loss drugs in development.
Once all trials conclude, Lilly will move toward FDA/MHRA submission, likely in late 2026 or 2027.
Is Retatrutide the Future of Weight Loss?
Retatrutide’s Phase 3 debut has changed expectations for what medical weight loss can achieve. The sheer magnitude of fat loss, which approaches bariatric surgery levels, and the improvements in pain and mobility create a strong case that this drug could define the next era of obesity treatment.
But tolerability will be a crucial factor. Some people will respond extraordinarily well. Others may struggle with the side effects or the rapid pace of weight loss.
Retatrutide may therefore become a drug best reserved for people who:
- Have high BMIs
- Need major weight reduction
- Have obesity-related complications
- Can be closely monitored
If approved, it will almost certainly reshape treatment guidelines and expectations for what’s possible with medication.
What This Means for Dieters
This may become the strongest weight-loss drug ever available
If you’ve struggled to lose weight even with lifestyle changes or current GLP-1 medications, retatrutide could offer levels of fat loss previously seen only with bariatric surgery.
It could dramatically improve mobility
Many people with obesity experience knee or back pain that limits exercise. Retatrutide’s improvements in osteoarthritis pain could help people become more active and maintain results long-term.
Side effects will matter a lot
Retatrutide isn’t just stronger, its side effects are notable, too. This may mean:
- Some people will need slower dose escalation
- Patients with lower BMIs may not tolerate it well
- Doctors will need to monitor symptoms closely
It could reshape treatment pathways
If approved by the FDA and MHRA, retatrutide may be recommended for:
- People needing large, rapid weight loss
- Those with severe obesity
- Patients with obesity-related complications
More moderate cases may still be better suited to GLP-1-only or dual-agonist medications.
Retatrutide is not yet available
The earliest timeframe for approval is 2026–2027, after the final Phase 3 trials conclude. That means more safety and long-term data are still on the way.
Medical Disclaimer
NowPatient has taken all reasonable steps to ensure that all material is factually accurate, complete, and current. However, the knowledge and experience of a qualified healthcare professional should always be sought after instead of using the information on this page. Before taking any drug, you should always speak to your doctor or another qualified healthcare provider.
The information provided here about medications is subject to change and is not meant to include all uses, precautions, warnings, directions, drug interactions, allergic reactions, or negative effects. The absence of warnings or other information for a particular medication does not imply that the medication or medication combination is appropriate for all patients or for all possible purposes.