The U.S. Food and Drug Administration (FDA) has approved the Wegovy pill, marking a historic milestone as the first oral GLP-1 medication for weight loss. This groundbreaking approval on December 22, 2025, offers millions of Americans struggling with obesity a convenient daily pill alternative to weekly injections.
What is the Wegovy Pill?
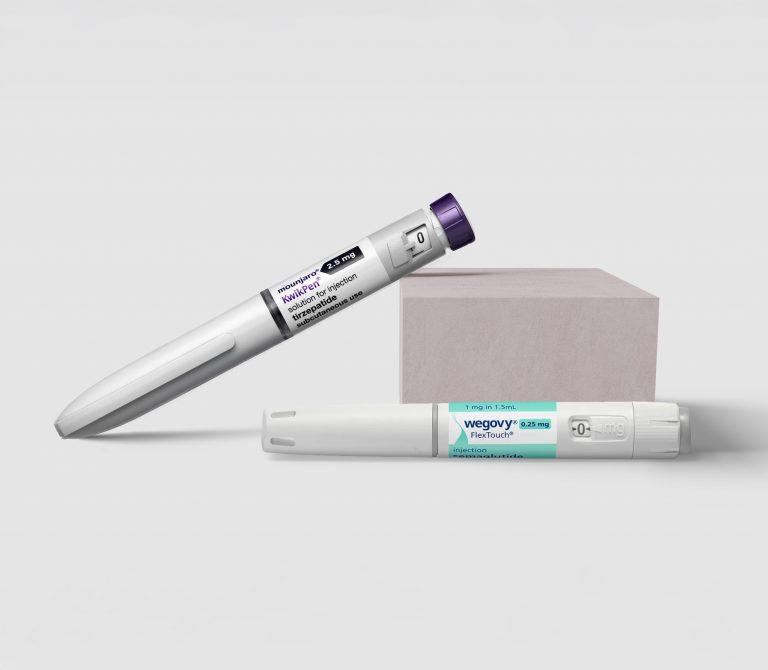
The Wegovy pill is an oral formulation of semaglutide, the same active ingredient found in the injectable versions of Wegovy and Ozempic. Manufactured by Novo Nordisk, this once-daily 25 mg tablet represents a significant advancement in obesity treatment, providing patients with a needle-free option for managing their weight.
Unlike its injectable counterpart, which requires weekly subcutaneous injections, the Wegovy pill is taken orally each day. This offers a more accessible treatment pathway for individuals who may have needle anxiety or prefer the convenience of oral medication.
FDA Approval Details
The FDA granted approval based on results from the OASIS clinical trial programme and the SELECT cardiovascular outcomes trial. The Wegovy pill has been approved for two primary indications. First, it is indicated for chronic weight management in adults with obesity (BMI ≥30 kg/m²) or overweight (BMI ≥27 kg/m²) with at least one weight-related comorbidity, when used alongside a reduced-calorie diet and increased physical activity. Second, it has been approved to reduce the risk of major adverse cardiovascular events (MACE), including death, heart attack, and stroke, in adults with established cardiovascular disease who also have obesity or overweight.
Clinical Trial Results: How Effective is the Wegovy Pill?
The OASIS 4 clinical trial demonstrated impressive results for the oral semaglutide formulation. The study enrolled 307 adults with obesity or overweight and followed them for 64 weeks.
Participants who adhered to treatment achieved an average weight loss of 16.6% of their body weight, compared to just 2.2% in the placebo group. Additionally, one in three patients experienced weight loss of 20% or greater. These results are comparable to the injectable Wegovy, which showed approximately 15% average weight loss in its pivotal trials.
The trial also evaluated a treatment policy analysis that included all participants regardless of adherence, which showed 13.6% average weight loss – still significantly higher than placebo.
How to Take Wegovy Pills
The Wegovy pill has specific dosing requirements that patients must follow for optimal absorption and effectiveness.
Patients should take the pill first thing in the morning on an empty stomach with no more than 4 ounces (approximately 120 ml) of plain water. After taking the medication, patients must wait at least 30 minutes before eating, drinking other beverages, or taking other oral medications.
The treatment follows a gradual dose escalation schedule to minimise gastrointestinal side effects. The starting dose is 1.5 mg, with increases over several weeks until reaching the maintenance dose of 25 mg daily.
Wegovy Pill vs. Wegovy Injection: Key Differences
While both formulations contain semaglutide and produce similar weight loss outcomes, there are important distinctions between the pill and injection.
Regarding administration, the pill is taken once daily orally while the injection is administered once weekly via subcutaneous injection. The dosage also differs significantly with the pill containing 25 mg at the maintenance dose compared to 2.4 mg for the injection. The pill’s higher dose compensates for lower bioavailability (absorption into blood stream) when taken orally, as some medication is broken down during digestion before reaching the bloodstream.
Clinical efficacy is comparable between the two formulations, with the pill showing 16.6% weight loss versus approximately 15% for the injection. However, the pill requires strict adherence to empty-stomach dosing for optimal results.
Common Side Effects
The Wegovy pill shares a similar side effect profile with other GLP-1 medications. The most commonly reported adverse effects include nausea, vomiting, diarrhoea, constipation, abdominal pain, headache, and fatigue.
These gastrointestinal side effects are typically most pronounced during the dose escalation phase and tend to diminish over time as the body adjusts to the medication. The gradual dose increase is specifically designed to help minimise these effects.
Patients should consult their healthcare provider if side effects are severe or persistent, as dose adjustments or supportive care measures may be recommended.
Availability and Cost for Wegovy Pills
Novo Nordisk has announced plans for a full U.S. launch in early January 2026. The starting dose of Wegovy 1.5 mg will be available in pharmacies and through select telehealth providers beginning in early January.
For the initial dose, Novo Nordisk is offering savings programmes with a self-pay price of $149 per month which represents the most affordable self-pay option for a GLP-1 obesity medication to date. Pricing for higher maintenance doses will vary, and patients should check with their insurance providers regarding coverage.
The company has indicated that manufacturing is well underway at their North Carolina facilities to ensure adequate supply for the anticipated demand.
Who is a Candidate for Wegovy Pills?
The Wegovy pill may be appropriate for adults who meet certain criteria. Candidates include those with a BMI of 30 or greater (obesity), those with a BMI of 27 or greater with at least one weight-related health condition such as type 2 diabetes, high blood pressure, or high cholesterol, adults with established cardiovascular disease who would benefit from cardiovascular risk reduction, and individuals who prefer oral medication over injections.
Patients should discuss with their healthcare provider whether the Wegovy pill is appropriate for their individual circumstances, medical history, and treatment goals.
Important Contraindications and Warnings
Certain individuals should not take Wegovy pills. The medication is contraindicated in patients with a personal or family history of medullary thyroid carcinoma (MTC), those with Multiple Endocrine Neoplasia syndrome type 2 (MEN 2), individuals with known hypersensitivity to semaglutide or any of the excipients, and those who are pregnant or breastfeeding.
Additionally, patients with a history of pancreatitis, severe gastrointestinal disease, or diabetic retinopathy should exercise caution and discuss risks with their healthcare provider.
The Future of Oral GLP-1 Medications
The approval of the Wegovy pill signals a new era in obesity treatment. With approximately 100 million American adults living with obesity, the availability of an oral GLP-1 option could significantly expand access to these highly effective medications.
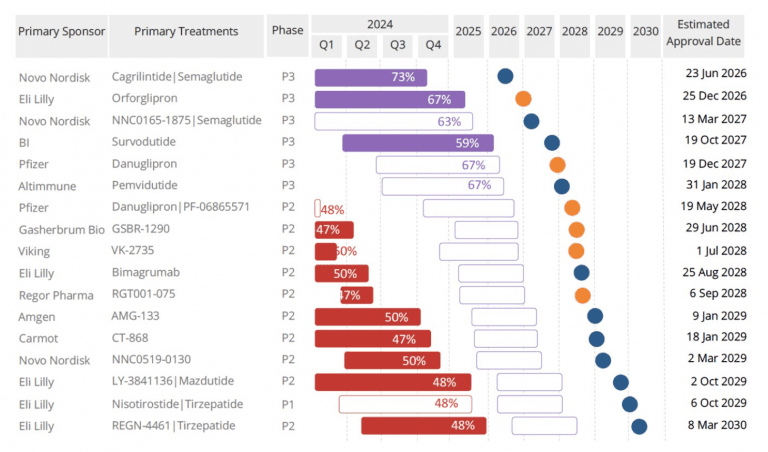
Competitor Eli Lilly is also developing an oral GLP-1 called orforglipron, which is currently under FDA review. Unlike the Wegovy pill, orforglipron can be taken without food or water restrictions, potentially offering even greater convenience. An FDA decision on orforglipron is expected by spring 2026.
Conclusion
The FDA approval of the Wegovy pill represents a significant advancement in obesity treatment, offering patients the first oral GLP-1 medication proven effective for weight management and cardiovascular risk reduction. With clinical trial results showing up to 16.6% average weight loss and a more convenient once-daily oral dosing option, the Wegovy pill provides a valuable new tool in the fight against obesity.
Patients interested in the Wegovy pill should consult with their healthcare provider to determine if this medication is appropriate for their weight management goals and overall health needs. As with all prescription medications, individual results may vary, and treatment should be part of a comprehensive approach that includes dietary modifications and increased physical activity.
Medical Disclaimer
NowPatient has taken all reasonable steps to ensure that all material is factually accurate, complete, and current. However, the knowledge and experience of a qualified healthcare professional should always be sought after instead of using the information on this page. Before taking any drug, you should always speak to your doctor or another qualified healthcare provider.
The information provided here about medications is subject to change and is not meant to include all uses, precautions, warnings, directions, drug interactions, allergic reactions, or negative effects. The absence of warnings or other information for a particular medication does not imply that the medication or medication combination is appropriate for all patients or for all possible purposes.